A group from Institut für Biochemie, Department für Chemie, Universität für Bodenkultur, Wien, Austria, etc. has reported about recognition of N-glycans of the porcine whipworm by the immune system.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10542551/
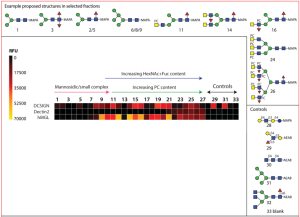
In this work, a natural glycan microarray using N-glycans (27 species) from porcine nematode parasite Trichuris suis was developed, and the interactions of these glycans with C-type lectins (DC-SIGN, Dectin-2, MGL), etc. were explored. Obtained N-glycans were fucosylated LacdiNAc motifs (bi/tri/tetra-antennary) with and without phosphorylcholine moieties and phosphorylcholine-modified oligomannose structures.
DC-SIGN recognises a rather wide range of oligomannosidic and fucosylated ligands, in the present study, its binding correlated generally with the occurrence of Man5-9GlcNAc2 in the relevant fractions.
Dectin-2 binding was lower on the array relativeto other innate immune system lectins.
MGL bound the majority of fractions, regardless of the presence of phosphorylcholine-modifications of the putative LacdiNAc-containing ligands.