A group from Amsterdam UMC, etc. has reported on mechanisms of immune modulations in pancreatic ductal adenocarcinoma (PDAC) through aberrant glycan modification, especially upregulated α2-3 sialic acids.
https://www.nature.com/articles/s41467-021-21550-4.pdf
PDAC is surrounded by dense fibrotic stroma and suppressive immune cells, mostly of the myeloid lineage such as dendric cells and macrophages (called TME). It has been known that aberrant glycosylation changes in PDAC are upregulation of sialyl Lewisx, sialyl Lewisa, truncated O-glycans, increased branched and fucosylated N-glycans, and are related to tumor cell proliferation, invasion, metastasis, and inflammation. However, their detailed link to the immune cell function in PDAC is still unknown.
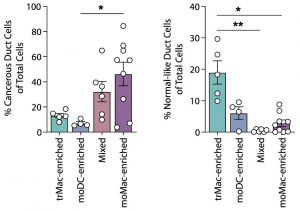
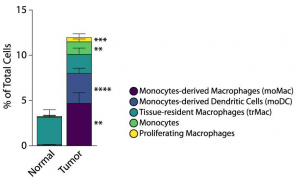
Authors have deep dived into the link between the aberrant sialylation and immune cells, and further its relationship with survival rate of PDAC. Composition of myeloid linkage cells composing TMA is greatly different between PDAC and healthy tissues. In PDAC, monocyte-derived macrophages (moMac) and monocyte-derived dendritic cells (moDC) accounted for the majority of the composition. Interestingly, patients with a higher presence of moMac over moDC had shorter survival, and the other myeloid populations did not correlate with the survival rate.
It was clearly shown that upregulated α2-3Sia bound to Siglec-7 and Siglec-9 expressed on myeloid cells, and drove the differentiation of monocytes to moMac with upregulation of CD206. Further, these changes induced upregulation of immune check point molecule PD-L1 and secretion of immunosuppressive cytokines such as IL-10.