Why SARS-CoV-2 (20I/501Y. V1) UK Variant does increase its infectability?
A group from Anschutz Medical Center, University of Colorado etc. has analyzed why does the SARS-CoV-2 (20I/501Y. V1) UK variant increase its infectability? using a molecular docking method.
https://www.biorxiv.org/content/10.1101/2021.02.02.428884v1.full
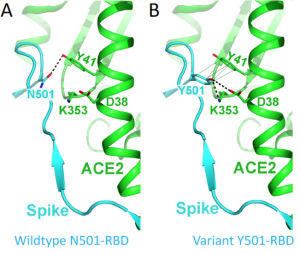
Comparing with the wild type N501, RBD of the mutant Y501 binds to ACE2 about 10 times more tightly, because a new hydrogen bond is formed between RBD and ACE2 (bold dashed line in the figure below), and there is a ring-ring interaction (light dashed line in the figure below).