Rhizosphere microbiota of Fritillaria ussuriensis (in the lily family Liliaceae) at different health levels
A group from College of Forestry, Northeast Forestry University, Harbin, China, etc. has reported about changes in rhizosphere microbiota of Fritillaria ussuriensis at different health levels.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8796711/
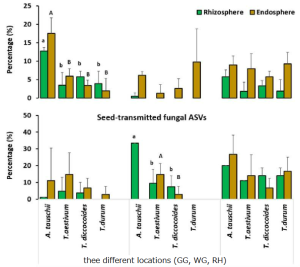
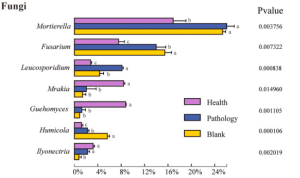
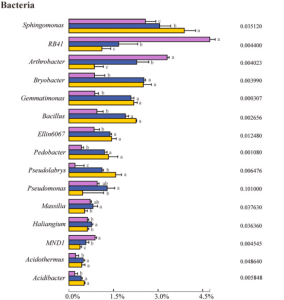
The difference in the composition of the soil microbial community at different health levels of Fritillaria ussuriensis, the top 7 fungal and 15 bacterial genera with high abundances were selected from the fungal and bacterial communities in healthy(H), pathologic(P), and blank(B) samples.
The difference in the relative abundance at the genus level indicated that Mortierella, Fusarium, Leucosporidium, Mrakia, Guehomyces, Humicola, and Ilyonectria were members of the fungal genera with higher abundances, among which Mortierella exhibiting the highest abundance (22.86%). Compared with the healthy sample, the abundance of Mortierella increased significantly in diseased samples (P and B). The abundances of Fusarium and Humicola increased significantly with increasing the severity of disease (H →P →B), with the blank sample having the highest abundance (15.49% and 5.60%, respectively). On the contrary, the abundances of Mrakia and Guehomyces decreased significantly, with the highest abundance in the healthy sample and more abundance and variation in the bacterial community compared with the fungal community.
In the case of rhizobacteria, the highest abundances of RB41 (4.74%) and Arthrobacter (3.30%) were found in the healthy sample. With increasing the severity of disease (H →P →B), the abundance decreased significantly. The relative abundances of Sphingomonas, Bryobacter, Gemmatimonas, Bacillus, Ellin6067, Pedobacter, Acidothermus, and Acidibacter in diseased samples (P and B) were significantly higher than that in healthy samples. Furthermore, the highest abundances of Bryobacter, Acidibacter, Pseudomonas, Massilia, and Haliangium were found in pathologic samples.

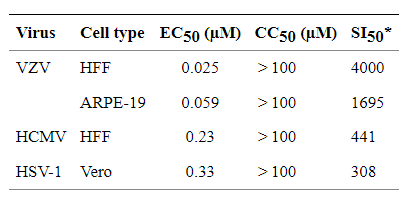 SI (selectivity index) calculated as CC50/EC50
SI (selectivity index) calculated as CC50/EC50