A group from Department of Immunobiology, Yale University School of Medicine, New Haven, CT, USA, etc. has reported that SARS-CoV-2 viral replication in human macrophages enhances an inflammatory cascade.
https://www.biorxiv.org/content/10.1101/2021.09.27.461948v1
In order to identify SARS-CoV-2 infected cells, authors used MISTRG6-hACE2 mice (humanized-ACE2 mice). As expected, SARS-CoV-2 viral RNA was detected in epithelial cells, the main targets of SARS-CoV-2 infections. Surprisingly, however, human immune cells also had similar levels of viral RNA. To better visualize and characterize these cells, MISTRG6-hACE2 mice were infected with a reporter strain of virus, SARS-CoV-2-mNG19. The majority of epithelial cells (EPCAM+) in bronchioalveolar lavage (BAL) but only a small proportion of total lung epithelial cells were infected with SARS-CoV-2 as measured by mNG expressing cells. Human immune cells showed a clear mNG signal, and mNG+ human immune cells were predominantly human macrophages.
Since macrophages are phagocytic, it is needed to address whether the SARS-CoV-2 viral RNA in these cells replicates in these cells or is simply acquired by phagocytosis of infected cells or debris. To achieve this, we first characterized the mNG signal in human macrophages from MISTRG6 mice that were not transduced with hACE2. In these mice, epithelial cells were not infected with SARS-CoV-2. These mice had however similar levels of mNG signal in the human macrophages of AAV-ACE2 mice, arguing that viral uptake by human macrophages is independent of infected epithelial cells. To determine whether SARS-CoV-2 replicates in human macrophages, the key characteristics of viral replication, viral replication products, subgenomic RNA, double stranded RNA (dsRNA), and the key replicative enzyme RNA dependent RNA polymerase (RdRp) were evaluated.
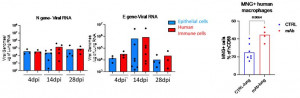
(1) Quantified genomic and subgenomic viral RNA in mNG+ vs mNG- epithelial or human immune cells at 4dpi or 14dpi. Only mNG+ epithelial cells and mNG+ human immune cells showed detectable subgenomic viral RNA. The levels of subgenomic RNA in human immune cells and epithelial cells were similar, suggesting a similar extent of viral replication in human immune cells and epithelial cells.
(2) Stained for dsRNA in mNG+ cells to determine whether the mNG signal colocalized with dsRNA (an exclusive product of viral replication). As expected, mNG and dsRNA were detectable and colocalized in both epithelial cells and in macrophages.
(3) Visualized viral RdRp in human macrophages.
In addition, the existence of antibody mediated viral uptake by macrophages was confirmed by treating infected mice with monoclonal antibodies against Spike protein of SARS-CoV-2, starting at 35 hours post infection (hpi). It was clearly shown that monoclonal antibody treatment increased the percentage of mNG+ macrophages in lungs.