A group from Basque Research & Technology Alliance (BRTA), Chemical Glycobiology Group, CIC bioGUNE, Bizkaia, Spain, etc. has reported that solution NMR and surface-based microarray studies provide different results on the molecular recognition features of LSECtin toward bi-antennary N-glycans.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9615123/
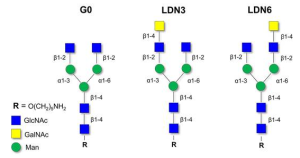
The molecular recognition features of LSECtin (CLEG4G) toward asymmetric N-glycans have been scrutinized by NMR and compared to those occurring in glycan microarrays. Strikingly, NMR studies confirmed that both asymmetric LDN3 and LDN6 N-glycans are recognized by LSECtin with similar affinities in solution, which is different in contrast to the results obtained when those glycans are presented on microarrays, where only LDN6 was efficiently recognized by the lectin.
Molecular recognition details differ from solution state to surfaces. Which one is closer to those existing in nature? Glycans are usually exposed on cell surfaces as part of glycoconjugates forming the glycocalyx. It is tempting to propose that the studies conducted using arrays are closer to those taking place on cell surfaces. However, the surface of slide glass is compretely different from actual cell surface glycocalyxes. Also, the length and chemical nature of the linkers used to attach the ligands to surfaces, and the composition of the solid support itself, could also influence the final outcome and the interpretation of the obtained results.