A group from Department of Chemistry, Massachusetts Institute of Technology, MS, USA, etc. has developed mannosylated macromolecules specific to each human mannose-binding lectins.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8549053/
Carbohydrate-binding proteins (lectins) play vital roles in cell recognition and signaling, including pathogen binding and innate immunity. Thus, targeting lectins, especially those on the surface of immune cells, could advance immunology and drug discovery. Lectins are typically oligomeric; therefore, many of the most potent ligands are multivalent. An effective strategy for lectin targeting is to display multiple copies of a single glycan epitope on a polymer backbone; however, a drawback to such multivalent ligands is they cannot distinguish between lectins that share monosaccharide binding selectivity (e.g., mannose-binding lectins such as DC-SIGN, DC-SIGNR, MBL, SP-D, langerin, dectin-2, mincle, and DEC-205) as they often lack molecular precision.
Authors developed β-mannosylated glyco-IEGmers specific to each lectins with mannose binding specificity.
To generate the target mannosylated glycomacromolecules, iterative exponential growth (IEG) cycles beginning from (R)- or (S)-glycidyl propargyl ether (GPE, > 99% ee) were conducted to yield oligo/polytriazoles with precisely 8, 16, or 32 allyl side chains. Macromolecules were produced with three different overall absolute configurations: all (R) (“isotactic”), all (S) (“isotactic”), and alternating (R-alt-S) (“syndiotactic”). To append the mannose residues, these IEGmers were exposed to pure β-thiomannose sodium salt under UV light (λ = 365 nm).
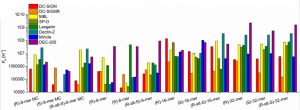
The binding affinity changed from sample to sample over several orders of magnitudes. For example, KA values for DC-SIGN ranged from approximately 1 × 104 for (S)-8mer to about 1 × 108 for (R)-16mer. For dectin-2, it also ranged from ∼1 × 104 to ∼1 × 108. DEC-205 bound all of the glyco-IEGmers with high KA values in the range of ∼1 × 106 to 1 × 109, with the highest KA of all pairwise interactions tested (2.2 × 109).
This wide range in affinity is impressive as the mannose binding epitopes presented was identical among these lectins.