A group from Far Eastern Branch of Russian Academy of Sciences, Vladivostok, Russia has reported on a new lectin, GYL, extracted from bivalve Glycymeris yessoensis.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8466245/
A lectin named GYL is a dimeric protein with a molecular mass of 36 kDa, as established by SDS-PAGE and MALDI-TOF analysis, consisting of 18 kDa subunits linked by a disulfide bridge.
The peptide sequence of GYL showed no significant homology with other lectins listed in the BLAST, however, the National Center for Biotechnology Information (NCBI) Conserved Domain Search program identified conserved EPN (Glu-Pro-Asn) and WND (Trp- Asn- Asp) motifs in the GYL peptide sequence, which are characteristic of the CRD of C-type lectins (CTL).
GYL was widely expressed throughout the tissues of healthy clams, however, levels of the lectin in the hemolymph and mantle were found to be 3.5- and 2.4-fold greater, respectively, than that found in the gonads.
GYL agglutinated all erythrocytes tested, suggesting that this activity is not highly specific. Although the detailed glycan binding specificity is not clearly shown, it was found that GYL preferentially bound peptidoglycan (PGN) and LPS expressed on bacteria cell walls, but had little binding activity toward β-1,3-glucan and mannan, which are usual components of the yeast cell wall.
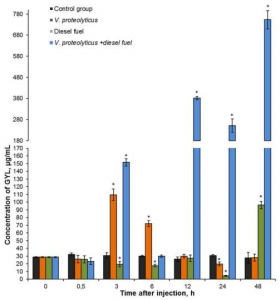
Taking these things into consideration, it was suggested that GYL plays an important role in the immune defense of the clam against pathogenic microorganism infections. Temporal expression levels of GYL in the hemolymph following V. proteolyticus stimulation and diesel fuel exposure were investigated. Amazingly, the expression levels of lectin increased about 25-fold at 48 h post-injection, suggesting that bacterial infection and anthropogenic factors had a significant effect on the clam, causing them to increase the synthesis of GYL as a protective molecule.