A new exosome marker for Alzheimer’s disease was discovered
A group of AIST and Kyoto Univ. has discovered a high-performance novel exosome marker as a novel marker for Alzheimer's disease.
https://pubmed.ncbi.nlm.nih.gov/33345458/
They found that in patients with Alzheimer's disease, there was a significant difference in glycan modification of exosomes compared to healthy people, and the exosomes were highly high mannosylated.
Using a high mannose specific lectin, its lectin blotting revealed a very strong band around 80 kDa, which was identified as a marker of exosomes, CD61.
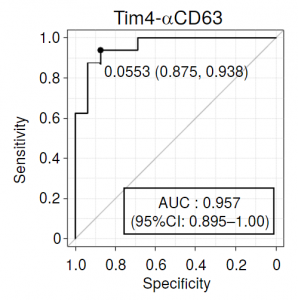
A sandwich assay (Tim4-αCD63) assembled with an antibody to the exosome marker CD63 and Tim4 (T cell immunoglobulin and mucin domain-containing protein 4) showed the highest discriminating ability among (CD61, CD41, CD63, and CD9), and the obtained AUC reached 0.957.