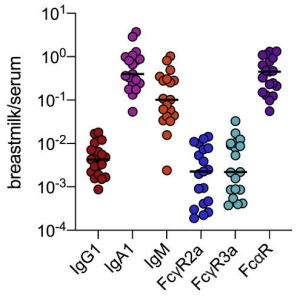
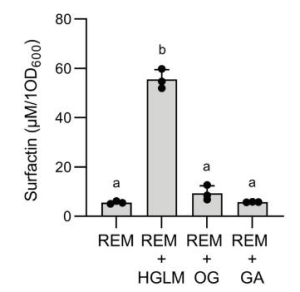
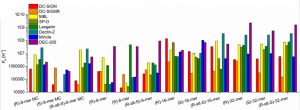
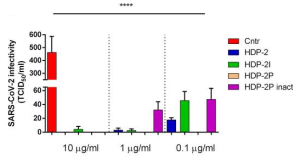
Antibody response induced by a 3rd boost SARS-CoV-2 vaccination: after 8 months from the 2nd vaccination
A group from The Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Kunming, Yunnan, China, etc. has reported on antibody response induced by a third boost dose of inactivated SARS-CoV-2 vaccine.
https://pubmed.ncbi.nlm.nih.gov/34666622/
In this study, 53 volunteers, who joined in the development and production of inactivated COVID-19 vaccines (it is not sure what company developed the relevant ones, though), received two doses (at 0 and 28 days) of the vaccines in 2020, and they received a 3rd dose 8 months after the 2nd dose recently.
At 0 days, 5 days, 7 days, and 14 days after the 3rd dose, blood was collected from 6 volunteers for the evaluation. It was found that both the anti-S antibody and neutralizing antibody against the original Wuhan strain gradually increased after 5 days, and the positive conversion rate of antibodies reached 100% at 14 days. Interestingly, the memory of IFN-γ-T cells against S, N, M, O antigens of SARS-CoV-2 can be quickly awakened after the 3rd dose.
These results indicate that although the neutralizing antibodies gradually decrease after two doses of inactivated vaccines, the antibody response could be awakened quickly by the 3rd vaccination and the T cell immune memory is still active even after 8 months from the 2nd vaccination.