A group of Institute for Glycomics, Griffith University, Gold Coast, QLD 4222, Australia has reported the importance of glycan-glycan interactions in HIV infection.
Host glycocalyx captures HIV proximal to the cell surface via
Current understanding of the roles of HIV glycans include: (1) to protect HIV from immune recognition; (2) to stabilize the trimeric envelope structure; and (3) to mediate trans-infection of T lymphocytes via electrostatic glycan-lectin (host proteinaceous lectin) interactions.
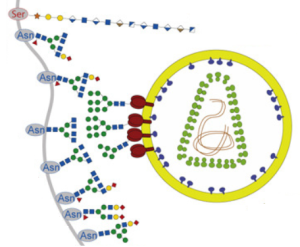
As is well know, all HIV Envelops are heavily glycosylated with variable N-linked glycans distributed across ~30 N-linked glycosylation sites, and many of these sites are primary occupied with oligomannose N-glycans, including Man5-9GlcNAc2-Asn and Man3GlcNAc2-Asn core structures.
While much is known about HIV entry, the initial interactions between virus and cell prior to HIV envelope-CD4 receptor-dependent interactions is rather unclear.
In this report, it was shown that glycan-glycan interactions could initiate HIV-cell contacts, that is, HIV- and host cell-glycan interactions via HIV oligomannose, Man5 (Manα1-3Manα1-6[Manα1-3]Man; a representative terminal HIV N-glycan structure, and host cell GlcNAc could potentiate HIV-host cell attachment.
So, it can be said that glycan-glycan interactions are emerging as a new class of high-affinity biomolecular interactions in virus infection.