Efficacy of Camostat Mesilate (TMPRSS2 inhibitor) for COVID-19 was disappointing
A group from Aarhus University Hospital, Denmark, etc. has reported on efficacy of Camostat Mesilate (which is a TMPRSS2 inhibitor) for COVID-19.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8060682/
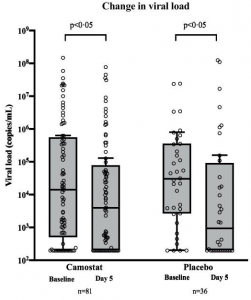
Authors have investigated it through a double-blind, randomized, placebo-controlled trial. The hypothesis behind it was that TMPRSS2 inhibition would block SARS-CoV-2 replication in infected patients leading to reduced viral loads, and that this in turn would lower the risk of hyper-inflammation and prevent disease progression. However, the results from the double-blind randomized placebo-controlled trial showed that camostat mesilate treatment did not significantly improve time to clinical improvement, the risk of intubation or death, time to discontinuation of supplemental oxygen, or any other efficacy outcomes among patients hospitalized with COVID-19.
Although the dose of camostat mesilate was not optimized in this trial, but Blog Admin feels that the famous infection passway through the ACE2-TMPRSS2 initiation is not major in pulmonary epithelial cells, but there might be other infection passways through C-type lectins expressed on immune cells and phagocytosis, and so forth.