A group from University of British Columbia, etc. has reported studies on molecular structures at the binding site of the SARS-CoV-2 spike protein of N501Y variant and ACE2 receptor with using a cryo-electron microscopy.
https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3001237
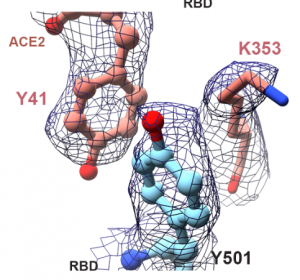
The overall binding structure at the binding site of ACE2 and SARS-CoV-2 is almost identical to that of the unmutated version, with the exception of some local rearrangements. The aromatic ring of Y501 is sandwiched between Y41 and K353 of the ACE2 receptor, and Y501 in the SARS-CoV-2 spike protein and Y41 in the ACE2 receptor form a perpendicular y-shaped π–π stacking interaction. This could be a reason of the increased infectivity.
The same conclusion from other researchers as this was already blogged.