A group from Institute of Physiology, University of Zurich, Zurich, Switzerland has reported about lectin cytotoxicity.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8866831/
While targeting surface glycans by lectins is a promising approach for cancer therapies, lectin-based approaches (lectin alone or in a combination with other therapeutic agents) are still at an early stage of development. The development of efficient therapies based on glycan targeting requires a deep understanding of the mechanisms of cell death induced by lectins on target tumor cells. It has been known that specific lectins induce distinct modes of cell death in different type of tumor cells. Wheat germ agglutinin (WGA), for example, induces apoptosis in melanoma and leukemic cell, whereas it kills cervical carcinoma cells through paraptosis-like cell death.
In this report, using murine adenocarcinoma cells (MC-38) and young adult mouse colon cells (YAMC), the multiple cell death pathways (apoptosis, necroptosis, proptosis, paraptosis, and autophagy-dependent cell death) activated in response to treatment of cells with lectins (MAL I, MAL II, SNA, AAL, WGA, and ECL) were studied with targeting different glycan structures. In order to characterize the signaling pathways mediating cell death induced by the cytotoxic lectins, a panel of MC-38 cells with knockouts in genes involved in cell death responses was used.
Knockouted genes are as follows;
pro-apoptotic proteins BCL2 antagonist/killer 1 (BAK1) and BCL2 associated X (BAX), which mediate intrinsic apoptosis, the intrinsic apoptosis signaling cascade is activated in response to various internal cell stress factors, such as DNA damage.
Fas-associated via death domain protein (FADD), which mediates extrinsic apoptosis, the extrinsic apoptosis pathway is induced in response to activation of cell death receptors, followed by formation of the death-inducing signaling complex that includes FADD and pro-caspase-8, which cleaves the executioner caspase-3.
tumor necrosis factor receptor type 1-associated death domain protein (TRADD), receptor-interacting serine/threonine protein kinases 3 (RIPK3), mixed lineage kinase domain-like protein (MLKL) and caspase-8 (CASP8), which mediate caspase-independent necroptosis.
caspase-1 (CASP1) and gasdermin D (GSDMD), which mediate pyroptosis, CASP1 is activated by the inflammasome in response to various microbial infections and non-infectious stimuli. The pathway leads to GSDMD cleavage, which embeds in the plasma membrane and forms pores that disrupt ionic gradients and facilitate water influx, hence leading to cell swelling and osmotic lysis.
Results:
The inactivation of the BAX/BAK1 complex decreased the cytotoxic response induced by WGA, MAL I and AAL treatment. The decrease in cytotoxicity by more than 50% was similar to the effect achieved in cells treated with cisplatin, which is a classical trigger of apoptosis. By contrast, the inactivation of FADD did not impacted the cell death mediated by WGA, MAL I and AAL.
The inactivation of TRADD, another adaptor molecule required for activation of apoptosis and necroptosis downstream of tumor necrosis factor receptor 1 (TNFR1), decreased cell death in cells treated with MAL I, but not when WGA and AAL was added. The loss of MLKL decreased the cytotoxic effect of WGA, MAL I and AAL, indicating the contribution of the necroptosis pathway in cell death induced by these lectins.
Inactivation of either CASP1 or GSDMD showed only a minor decrease in MAL I-mediated cytotoxicity, supporting a partial involvement of pyroptosis in response to MAL I treatment.
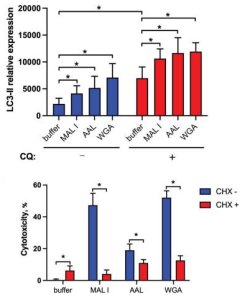
In addition to the induction of apoptosis, many lectins also up-regulate autophagy sometimes resulting in autophagy-dependent cell death. LC3-II is associated with autophagosome membranes. Treatment with MAL I, AAL and WGA up-regulated LC3-II in lysates from cells treated for 6 h with lectins. The increase in LC3-II levels indicated that cell death induced by MAL I, AAL and WGA was probably initiated through activation of the autophagic/lysosomal response rather than the classical apoptotic mitochondrial pathway. Actually by adding cycloheximide (CHX), is known to block starvation-induced autophagy, the cytotoxic response of the three lectins was significantly reduced.