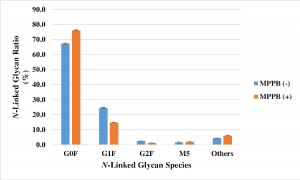
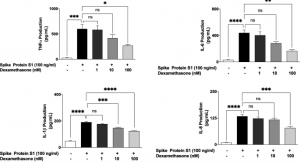
Vaccine effectiveness of Pfizer-BioNTech or Moderna vaccine against COVID-19
CDC COVID-19 Response Team has reported about the vaccine effectiveness of Pfizer-BioNTech or Moderna vaccine.
https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7018e1-H.pdf
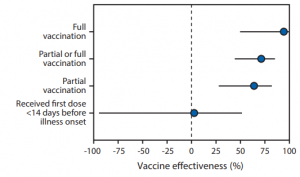
The team has reported that adjusted vaccine effectiveness for full vaccination using Pfizer-BioNTech or Moderna vaccine was 94% (95% CI = 49%–99%), and that for partial vaccination was 64% (95% CI = 28%–82%).
In this study, the vaccine effectiveness was assessed among 417 adults (including 187 case-patients and 230 controls) aged ≥65 years who admitted with COVID-19–like illness and confirmed by RT-PCR during January 1, 2021–March 26, 2021. Case-patients were those who received one or more positive test results for SARS-CoV-2. Patients meeting eligibility criteria who received negative SARS-CoV-2 RT-PCR test results served as controls. Participants in this study were considered to have received COVID-19 vaccine doses based on official CDC vaccination record card etc. and by plausible self-report.
It should be noted that there was no significant effect for receiving the first dose of a 2-dose COVID-19 vaccine series within 14 days before illness onset (adjusted vaccine effectiveness = 3%, 95% CI = −94%–51%)