Universidad de Oviedo, Spain has developed aptamers binding to both the glycan and the near the peptide region of human prostate specific antigen (hPSA).
https://pubmed.ncbi.nlm.nih.gov/34094206/
To achieve a finer direction of the aptamers for integrating in the recognition both the glycan and the near peptide region of hPSA, Pholiota squarrosa lectin (PhoSL) was used as a specific lectin of α1-6 core-fucose, increasing the likelihood of obtaining aptamers specific for the glycosylation site of the protein.
The SELEX approach involved the immobilization of either hPSA or recombinant PSA (rPSA), which has no glycans, onto magnetic particles (MPs), using bovine serum albumin (BSA) as a blocking agent. Each SELEX round included a negative selection step with BSA–MPs to eliminate sequences that bind to BSA, followed by a counter-selection against rPSA-MPs to remove molecules that bind to the protein by regions different from the glycosylation site, and a positive selection step with hPSA–MPs to enrich the starting library in sequences that bind to the desired glycan moiety in the protein.
After these initial rounds, the pool was split to perform two distinct routes. Strategy A includes an extra more stringent counter-selection step by blocking the α1-6 core-fucose with PhoSL, removing molecules that remained bound to the blocked protein. Strategy B, in contrast, relies on the competitive elution of aptamers bound to the PhoSL binding site. Atrategy A leads to a decrease in the number of aptamers bound to hPSA, while the competitive elution with PhoSL produces a progressive increase in both population of hPSA-binders and their average binding affinity.
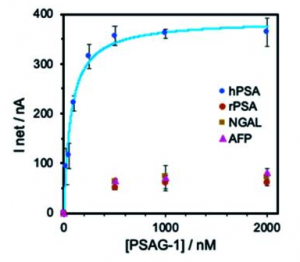
A figure below shows binding specificity of selected aptamer (PSAG-1)including two core-fucosylated glycoproteins: lipocalin-2 (NGAL) and α-fetoprotein (AFP). If more precise glycan structures specific to prostate caner could be recognized with this type of aptamers, it would be great.