A group from Technical University of Munich, School of Medicine, Neuherberg, Germany, etc. has reported about a novel monoclonal IgG1 antibody specific for α-Gal epitope.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9391071/
The alpha-Gal (α-Gal) epitope is a carbohydrate immunogen in humans that has relevance in allergy and xenotransplantation. Interestingly, antibodies of different isotypes against the α-Gal epitope are quite abundant in humans with IgG levels estimated to range between 1% to 0.1% of total plasma IgG with high variability between subjects and lowest abundance in individuals carrying the blood type B antigen. This observation is likely due to the structural similarity between the α-Gal epitope and blood type B antigen, which contains an additional fucose molecule on the second last galactose molecule. These human anti-α-Gal antibodies pose a challenge for xenotransplantation, in particular for pig organ transplantation, which was overcome to some extend with developing GGTA1 knockout (KO) pigs.
In this paper, the development of a novel IgG1 antibody called 27H8 was reported, which is highly specific for both synthetic and naturally occurring α-Gal epitopes. In order to generate a monoclonal antibody specific for the α-Gal epitope determining structure Gal-α1,3-Gal that is equally able to bind to the naturally occurring α-Gal epitope Gal-α1,3-Gal-β1,4-GlcNAc, α-galactosyltransferase knockout mice (Ggta1 KO) were immunized with Gal-α1,3-Gal-β1,4-GlcNAc coupled to ovalbumin as carrier protein (α-Gal-OVA).
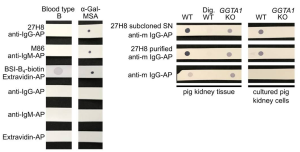
To verify the specificity, the 27H8 monoclonal antibody was compared to Bandereia simplifolica isolectin B4 (BSI-B4: GSL-I B4) and to the monoclonal IgM antibody M86, which are both widely used to detect the α-Gal epitope. BSI-B4 is specific for terminal α-galactose oligosaccharides and therefore recognizes also the blood group B antigen, which differs from the α-Gal epitope only in the addition of one fucose residue and is thus structurally very similar. To assess whether 27H8 also binds to the blood group B antigen we blotted lysates of whole blood from a type B donor on a membrane and applied the antibodies 27H8 and M86 or biotinylated BSI-B4 for detection. While BSI-B4 bound to the blood type B specimen as expected, neither 27H8 or M86 did. Next, it was investigated whether 27H8 also binds to natural α-Gal epitopes. As pig kidney is naturally rich in α-Gal and reactions in α-Gal allergic patients are severe after ingestion, it was also tested if 27H8 recognizes α-Gal in pig kidney lysates in a dot blot assay. 27H8 binding to wildtype (WT) pig kidney lysate was observed with strong staining intensity. However, there was no signal on a sample digested with α-Galactosidase (Dig. WT).