ACE2 and BSG/CD147 are involved in SARS-CoV-2 infection of human iPS cell-derived podocytes
A group from Department of Biomedical Engineering, Pratt School of Engineering, Duke University, Durham, NC, USA, etc. has reported that ACE2 and BSG/CD147 are involved in SARS-CoV-2 infection of human iPS cell-derived podocytes.
https://pubmed.ncbi.nlm.nih.gov/34816259/
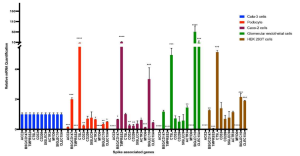
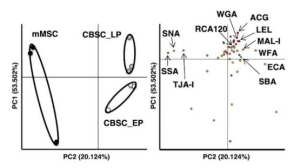
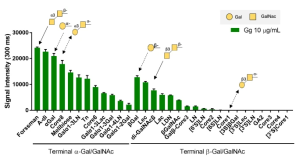
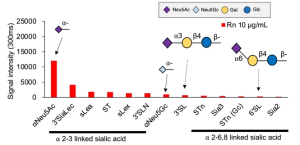
Human iPS cell-derived podocytes express many host factor genes (including ACE2, BSG/CD147, TMPRSS2, CTSL, CD33, DC-SIGN/CD209, SIGLEC9, SIGLEC10, ACTR3, CLEC10A) associated with SARS-CoV-2 binding and viral processing. It is known that proteases such as Transmembrane Serine Protease 2 (TMPRSS2) or cathepsin L (CTSL) promote fusion and internalization of the receptorviral spike complex. Human iPS cell-derived podocytes expressed lower levels of ACE2 and TMPRSS2 when compared to Calu-3, and both BSG/CD147 and CTSL were expressed at high levels as shown below.
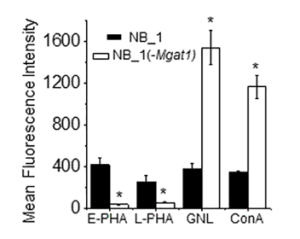
Nevertheless, podocytes derived from human iPS cells were directly infected with SARS-CoV-2 at low Multiplicity of Infection (MOIs) of 0.01 to 1, and intriguingly, there was significantly more viral uptake in the podocytes than Calu3 (p-value < 0.0001) and Caco-2 cells (p-value < 0.0001). Pre-treatment of infected podocytes with both anti-ACE2 and anti-BSG/CD147 antibodies at a concentration as low as 0.1 µg/ml significantly diminished viral uptake (p-value < 0.0001), suggesting that both ACE2 and BSG/CD147 are involved in SARS-CoV2 internalization in human iPS cell-derived podocytes.